$40.00 – $390.00
| CAS No.: | 128-57-4 |
| Molecular Formula | C₄₂H₃₈O₂₀ |
| Molecular Weight | 862.7g/mol |
| Purity | HPLC>98% |
| Chemical Family | Anthraquinones |
* Warning: BIORLAB’s Natural Compounds are for laboratory research purposes only.
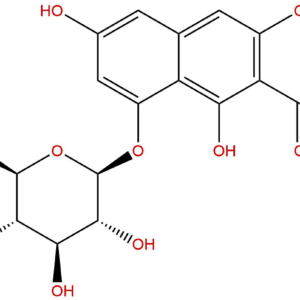
IUPAC Name: (9S)-9-[(9R)-2-carboxy-4-hydroxy-10-oxo-5-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-9H-anthracen-9-yl]-4-hydroxy-10-oxo-5-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-9H-anthracene-2-carboxylic acid
InChI: InChI=1S/C42H38O20/c43-11-23-31(47)35(51)37(53)41(61-23)59-21-5-1-3-15-25(17-7-13(39(55)56)9-19(45)27(17)33(49)29(15)21)26-16-4-2-6-22(60-42-38(54)36(52)32(48)24(12-44)62-42)30(16)34(50)28-18(26)8-14(40(57)58)10-20(28)46/h1-10,23-26,31-32,35-38,41-48,51-54H,11-12H2,(H,55,56)(H,57,58)/t23-,24-,25-,26+,31-,32-,35+,36+,37-,38-,41-,42-/m1/s1
InChIKey: IPQVTOJGNYVQEO-AIFLABODSA-N
Canonical SMILES: C1=CC2=C(C(=C1)OC3C(C(C(C(O3)CO)O)O)O)C(=O)C4=C(C2C5C6=C(C(=CC=C6)OC7C(C(C(C(O7)CO)O)O)O)C(=O)C8=C5C=C(C=C8O)C(=O)O)C=C(C=C4O)C(=O)O
Isomeric SMILES: C1=CC2=C(C(=C1)O[C@H]3[C@@H]([C@H]([C@@H]([C@H](O3)CO)O)O)O)C(=O)C4=C([C@@H]2[C@H]5C6=C(C(=CC=C6)O[C@H]7[C@@H]([C@H]([C@@H]([C@H](O7)CO)O)O)O)C(=O)C8=C5C=C(C=C8O)C(=O)O)C=C(C=C4O)C(=O)O
Synonyms: Sennoside B; 128-57-4; MLS002473001; CHEBI:34975; MFCD00151528; F887D1637W; (9R,9’S)-4,4′-dihydroxy-10,10′-dioxo-5,5′-bis(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-9,9′,10,10′-tetrahydro-[9,9′-bianthracene]-2,2′-dicarboxylic acid; SMR001397106; …
Botanical Source: Sennae Folium
Sennoside B is a bioactive anthraquinone glycoside with significant pharmacological properties. BIORLAB provides high-purity sennoside B isolated through advanced chromatographic techniques and validated analytical methods for pharmaceutical research applications.
Gastrointestinal Studies
Metabolic Research
Pharmaceutical Applications
For research use only. Not for human consumption or therapeutic use.
BIORLAB commits to excellence and reliability.
Your orders are shipped seamlessly between countries
You have the right to return your orders within 30 days.
Your payments are secure with our private security network.

For you to better choose the right compounds, and so the scientific research can be carried out smoothly, here are some FAQs.
Delivery times vary based on order size, We deliver small quantities of compounds within 2 working days, ranging from one week for up to 500 compounds to about 6 weeks for orders exceeding 5,000 compounds.
While our focus is on natural compounds, we can provide information on natural product-like synthetic compounds if requested.
Yes, we provide comprehensive analytical data and certificates of analysis for all our compounds.
Yes, we can re-supply active compounds in larger quantities and offer analogs for further testing if hits are discovered.
We offer a wide range of natural compounds including alkaloids, terpenoids, flavonoids, coumarins, peptides, glycosides, and nucleosides isolated from plants.
Our compounds undergo rigorous purification processes and are tested using advanced analytical methods such as NMR, mass spectroscopy, and in some cases, X-ray analysis to ensure >98% purity.
Yes, we offer custom isolation services for specific natural compounds based on client requirements.
We typically offer quantities ranging from 2 mg to 100 mg for screening purposes, with larger amounts available upon request.
We use various physicochemical analytical methods, including NMR (300-500 MHz) and mass spectroscopy, to confirm structures and stereochemistry.
BIORLAB is committed to providing highly competitive natural compounds and comprehensive solutions to customers around the world.






Get The Latest Updates and Promotion Information.
