Analytical Services
BIORLAB offers an integrated and comprehensive analytical service to ensure quality control to meet customers.
Analytical Service
Analytical services play a pivotal role in biotechnology, particularly in pharmaceutical development. They ensure compounds’ integrity, purity, and efficacy, thereby influencing regulatory compliance and quality assurance standards.
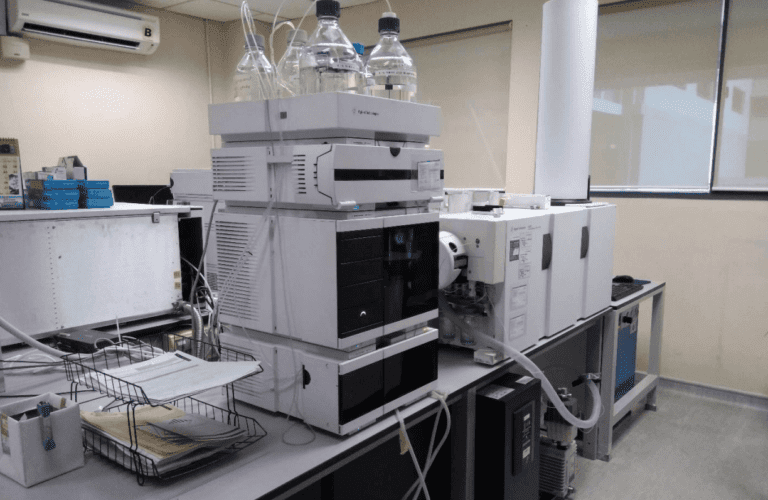
BIORLAB offers a comprehensive suite of analytical services, including High-Performance Liquid Chromatography (HPLC), Gas Chromatography (GC), Nuclear Magnetic Resonance (NMR), and more. These services cater to diverse needs in drug development, from initial analysis to quality control.
Analysis Services Range
At BIORLAB, our Analytical Services are designed to support every stage of drug development and manufacturing, ensuring precision, reliability, and compliance with stringent regulatory requirements. Our state-of-the-art laboratories and experienced team enable us to deliver accurate results that drive innovation and accelerate research outcomes.
- Content and purity determination: HPLC, GC, IC, NMR
- Determination of total organic impurities (total related substances): HPLC, GC
- Counter ion determination (organic, inorganic anions and cations): Ion chromatography, HPLC
- Determination of heavy metals: Pharmacopeial methods, atomic absorption
- Determination of enantiomers and diastereomers: Chiral chromatography, optical rotation determination
- Structural confirmation studies: Elemental analysis, mass spectrometry, NMR, FTIR, UV
- GC and GCMS analysis (headspace+direct injection)
- Residual solvent detection
- Routine physical and chemical detection (ROI, ICP-MS, IC, XRD, DVS, infrared, ultraviolet, melting point, optical rotation, moisture determination, titration, heavy metal residue detection, etc.)
- Thermal analysis (TGA, DSC)
- Physical and chemical constant test: LogP, LogD, PKa test.
Analytical Techniques
High-Performance Liquid Chromatography (HPLC) and Gas Chromatography (GC)
HPLC and GC are fundamental techniques for separating, identifying, and quantifying compounds within complex mixtures. These methods are pivotal in assessing drug purity, detecting impurities, and ensuring product consistency.
Mass Spectrometry (MS) and Nuclear Magnetic Resonance (NMR)
MS and NMR provide insights into molecular structures, facilitating precise characterization of compounds. These techniques are crucial in structural elucidation, metabolite identification, and quality assessment.
Micro ED structure analysis technology
Microcrystalline electron diffraction technology (Micro ED technology) is a technology that determines the three-dimensional spatial structure of crystals based on electron diffraction data obtained by cryo-TEM.
This technology uses high-energy electrons to replace traditional X-rays as a radiation source, increasing the efficiency of structural analysis by 10 times and the success rate by 1 time. Therefore, only a small amount of micro-nano (>0.1um) grains are needed to quickly and efficiently obtain high-resolution diffraction data, greatly reducing the requirements for sample shape, purity, and size.
Request Analytical Services
Applications in Drug Development
Quality Control in Drug Manufacturing
BIORLAB’s analytical services are instrumental in maintaining stringent quality control standards throughout the drug manufacturing process. This ensures that pharmaceutical products meet regulatory requirements globally, safeguarding patient safety and efficacy.
Our Advantages
- GMP Compliance: We ensure all our analytic testing services meet Good Manufacturing Practice standards.
- Method Development: Tailored methods for precise analysis from development to validation.
- Impurity Isolation: Expertise in isolating impurities from milligrams to multi-gram levels.
- Advanced Tools: Utilization of cutting-edge tools like LC/MS/MS, and NMR for precise identification.
- Stability Testing: Adherence to ICH guidelines for robust stability testing protocols.
FAQs
Our laboratories are equipped with a range of cutting-edge instruments, including HPLC, GC, NMR, and more, ensuring comprehensive analytical capabilities.
Our technical team comprises seasoned experts with extensive experience in biotech and pharmaceutical sciences, providing specialized knowledge and innovative solutions.
Yes, our facilities and workflows are designed to handle high-volume sample analysis efficiently, ensuring timely delivery without compromising accuracy.
We adhere to rigorous quality assurance processes and standards, including method validation and compliance with regulatory guidelines, to guarantee the accuracy and reliability of our analytical data.
Absolutely. We collaborate closely with clients to tailor our analytical services to meet specific project requirements, ensuring personalized solutions that address unique scientific challenges.